what structures are the bound ribosomes attached to
Click to view a research level microscope image, interpreted using CIMR GridPoint engineering science
Quick look:
A ribosome functions as a micro-machine for making proteins. Ribosomes are equanimous of special proteins and nucleic acids. The TRANSLATION of data and the Linking of AMINO ACIDS are at the heart of the protein production procedure. A ribosome, formed from two subunits locking together, functions to: (i) Interpret encoded information from the cell nucleus provided by messenger ribonucleic acid (mRNA), (two) Link together amino acids selected and collected from the cytoplasm by transfer ribonucleic acid (tRNA). (The lodge in which the amino acids are linked together is determined by the mRNA) and, (3) Export the polypeptide produced to the cytoplasm where it will form a functional protein.
Ribosomes are establish 'gratis' in the cytoplasm or jump to the endoplasmic reticulum (ER) to form rough ER. In a mammalian prison cell there can be equally many as ten million ribosomes. Several ribosomes can be fastened to the same mRNA strand, this structure is chosen a polysome. Ribosomes have but a temporary existence. When they have synthesised a polypeptide the ii sub-units split up and are either re-used or broken up.
Ribosomes can join up amino acids at a rate of 200 per minute. Small proteins can therefore be fabricated fairly rapidly only two to three hours are needed for larger proteins such as the massive 30,000 amino acid muscle poly peptide titin.
Ribosomes in prokaryotes use a slightly dissimilar procedure to produce proteins than do ribosomes in eukaryotes. Fortunately this divergence presents a window of molecular opportunity for set on by antibiotic drugs such every bit streptomycin. Unfortunately some bacterial toxins and the polio virus also utilize it to enable them to attack the translation mechanism.
For an overview diagram of protein production click here.
(The diagram will open up in a dissever window)
-
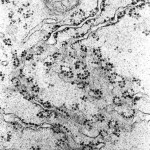
This is an electron microscope epitome showing office of the rough endoplasmic reticulum in a found root cell from maize. The dark spots are ribosomes.
(courtesy of Chris Hawes, The Research Schoolhouse of Biological science & Molecular Sciences, Oxford Brookes University, Oxford, UK)
A LONGER LOOK at Ribosomes:
Ribosomes are macro-molecular product units. They are composed of ribosomal proteins (riboproteins) and ribonucleic acids (ribonucleoproteins). The word ribosome is made from taking 'ribo' from ribonucleic acid and adding it to 'soma', the Latin discussion for trunk. Ribosomes can be leap by a membrane(s) but they are not membranous.
Ribosome: a micro-machine for manufacturing proteins
A ribosome is basically a very complicated but elegant micro-'automobile' for producing proteins. Each complete ribosome is constructed from ii sub-units. A eukaryotic ribosome is composed of nucleic acids and about eighty proteins and has a molecular mass of about four,200,000 Da. Nearly two-thirds of this mass is composed of ribosomal RNA and i tertiary of about 50+ different ribosomal proteins.
Ribosomes are found in prokaryotic and eukaryotic cells; in mitochondria, chloroplasts and leaner. Those found in prokaryotes are mostly smaller than those in eukaryotes. Ribosomes in mitochondria and chloroplasts are similar in size to those in bacteria. In that location are nearly ten billion protein molecules in a mammalian cell and ribosomes produce most of them. A rapidly growing mammalian cell can contain about 10 million ribosomes. [A single cell of East. Coli contains about xx,000 ribosomes and this accounts for about 25% of the total cell mass].
The proteins and nucleic acids that course the ribosome sub-units are fabricated in the nucleolus and exported through nuclear pores into the cytoplasm. The two sub-units are unequal in size and exist in this state until required for utilise. The larger sub-unit of measurement is well-nigh twice as large as the smaller one.
The larger sub-unit has mainly a catalytic function; the smaller sub-unit of measurement mainly a decoding one. In the large sub-unit ribosomal RNA performs the function of an enzyme and is termed a ribozyme. The smaller unit links up with mRNA and then locks-on to a larger sub-unit. Once formed ribosomes are non static units. When product of a specific poly peptide has finished the two sub-units separate and are and then usually broken down. Ribosomes have only a temporary existence.
Sometimes ribosome sub-units admit mRNA every bit soon as the mRNA emerges from the nucleus. When many ribosomes do this the structure is chosen a polysome. Ribosomes can part in a 'free' state in the cytoplasm simply they tin can also 'settle' on the endoplasmic reticulum to form 'crude endoplasmic reticulum'. Where there is rough endoplasmic reticulum the clan between ribosome and endoplasmic reticulum (ER) facilitates the further processing and checking of newly made proteins by the ER.
The Poly peptide Mill: site and services.
All factories need services such as gas, water, drainage and communications. For these to be provided there must a location or site.
Poly peptide production also needs service requirements. A site requiring the provision of services is produced in a modest ribosome sub-unit when a strand of mRNA enters through 1 selective fissure, and a strand of initiator tRNA through some other. This activeness triggers the pocket-sized sub-unit to lock-on to a ribosome large sub-unit to form a complete and active ribosome. The amazing process of protein product can now begin.
For translation and protein synthesis to take place many initiator and release chemicals are involved, and many reactions using enzymes take place. There are however general requirements and these have to exist satisfied. The list beneath shows the main requirements and how they are provided:
- Requirement: A prophylactic (contagion free) and suitable facility for the protein production procedure to accept place.
- Provision: this facility is provided by the two ribosomal sub-units. When the two sub-units lock together to grade the complete ribosome, molecules entering and exiting can only do and then through selective clefts or tunnels in the molecular structure.
- Requirement: A supply of data in a form that the ribosome can translate with a high caste of accuracy. The translation must be accurate in guild that the right proteins are produced.
- Provision: Information is supplied by the nucleus and delivered to the ribosome in the form of a strand of mRNA. When mRNA is formed in the nucleus introns (non-coding sections) are cut out, and exons (coding sections) are joined together past a process chosen splicing.
- Requirement: A supply of amino acids from which the ribosomal mechanism tin obtain the specific amino acids needed.
- Provision: Amino acids, mainly supplied from food, are usually freely bachelor in the cytoplasm.
- Requirement: A organisation that can select and lock-on to an amino acid in the cytoplasm and deliver it to the translation and synthesis site in the ribosome.
- Provision: Curt strands of transfer ribonucleic acid (tRNA) made in the nucleus and available in the cytoplasm act equally 'adaptor tools'. When a strand of tRNA has locked on to an amino acid the tRNA is said to be 'charged'. tRNA diffuses into the smaller ribosome sub-unit and each brusk tRNA strand volition deliver I amino acrid.
- Requirement: A means of releasing into the cytoplasm: (a) a newly formed polypeptide, (b) mRNA that has been used in the translating process, and (c) tRNA that has delivered the amino acid information technology was carrying and is now 'uncharged'.
- Provision: (a) when a newly formed peptide chain is produced deep inside the ribosome large sub-unit of measurement, information technology is directed out to the cytoplasm along a tunnel or cleft. (b) 'Used' mRNA leaves the smaller ribosome sub-unit through a tunnel on the side opposite to its point of entry. Movement through the ribosome is brought about past a one-style only, intermittent motion of the ribosome forth, and in the direction of, the incoming mRNA strand.(c) tRNA in the 'uncharged' state leaves via a tunnel in the molecular architecture of the ribosome large sub-unit.
The Protein Factory: What happens on the within?
– A look at the poly peptide production line that can join up amino acids at a rate of 200 per minute!
Now nosotros have considered the requirements and provisions needed for the protein production machine to operate, we can look at the inner workings.
Every bit mentioned earlier many detailed biochemical reactions take place in the ribosome and simply a brief outline is given hither to illustrate the concept.
(Delight as well see 'schematic of ribosome' at finish of section)
In the ribosome there are THREE STAGES and Three operational SITES involved in the poly peptide production line.
The three STAGES are (1) Initiation, (2) Elongation and (3) Termination.
The 3 operational or binding SITES are A, P and E reading from the mRNA entry site (conventionally the right mitt side).
Sites A and P span both the ribosome sub-units with a larger office residing in the ribosome large sub-unit of measurement, and a smaller function in the smaller sub-unit. Site East, the exit site, resides in the large ribosome sub-unit.
Table of bounden sites, positions and functions in a ribosome
(delight too run into schematic of ribosome at end of department)
| Bounden Site | mRNA strand entry site | Biological term | Master processes |
| Site A | 1st | Aminoacyl | Access of codon of mRNA & 'charged' strand of tRNA. Checking and decoding and start of 'handing over' one amino acrid molecule |
| Site P | 2d | Peptidyl | Peptide synthesis, consolidation, elongation and transfer of peptide chain to site A |
| Sitdue east Eastward | tertiary | Exit-to cytoplasm | Grooming of 'uncharged' tRNA for exit |
The 3 stages:
- Initiation. During this stage a small ribosome sub-unit links onto the 'start end' of an mRNA strand. 'Initiator tRNA' also enters the minor sub-unit. This circuitous then joins onto a ribosome large sub-unit of measurement. At the first of the mRNA strand there is a 'start translating' message and a strand of tRNA 'charged' with one specific amino acid, enters site A of the ribosome. Production of a polypeptide has now been initiated.For the tRNA not to be rejected the iii letter code group information technology carries (called an anti-codon) must friction match upwards with the three letter code group (called a codon) on the strand of mRNA already in the ribosome. This is a very of import part of the translation process and it is surprising how few 'errors of translation' occur. [In general the particular amino acid it carries is adamant by the three letter anticodon it bears, e.g. if the three alphabetic character code is CAG (Cytosine, Adenine, Yarduanine) and so it will select and transport the amino acid Glutamine (Gln)].
- Elongation.This term covers the menses between initiation and termination and information technology is during this time that the main part of the designated protein is made. The process consists of a serial of cycles, the total number of which is determined by the mRNA. 1 of the primary events during elongation is translocation. This is when the ribosome moves along the mRNA by one codon notch and a new bike starts.During the 'outset-up' process the 'initiation tRNA' volition take moved to site P (see schematic of ribosome at end of department) and the ribosome will accept admitted into site A, a new tRNA 'charged' with one amino acid.The 'charged' tRNA resides in site A until information technology has been checked and accepted (or rejected) and until the growing peptide chain attached to the tRNA in site P, has been transferred beyond by enzymes, to the 'charged' tRNA in site A. Hither one new amino acid is donated by the tRNA and added to the peptide concatenation. By this procedure the peptide chain is increased in length by increments of one amino acid. [The peptide bond formation betwixt the growing peptide chain and the newly admitted amino acrid is assisted past peptidyl transferase and takes identify in the big ribosome sub-unit. The reaction occurs betwixt tRNA that carries the nascent peptide chain, peptidyl-tRNA and the tRNA that carries the incoming amino acrid, the aminoacyl-tRNA]. When this has taken place the tRNA in site P, having transferred its peptide concatenation, and at present without any attachments, is moved to site E the exit site.Next, the tRNA in site A, consummate with a peptide concatenation increased in length by one amino acid, moves to site P. In site P riboproteins act to consolidate the bonding of the peptide chain to the newly added amino acid. If the peptide concatenation is long the oldest function will be moved out into the cytoplasm to be followed by the residuum of the chain as it is produced.The side by side cycle
With site A now empty translocation takes place. The ribosome moves on by a distance of one (3 letter) codon notch along the mRNA to bring a new codon into the processing area. tRNA 'charged' with an attached amino acid at present enters site A, and provided a satisfactory friction match of the mRNA codon and tRNA anti-codon is fabricated, the bike starts once again. This process continues until a termination stage is reached. - Termination. When the ribosome reaches the end of the mRNA strand, a last or 'cease of poly peptide code' bulletin is flagged upwards. This registers the finish of production for the detail poly peptide coded for by this strand of mRNA. 'Release factor' chemicals prevent whatever more amino acid additions, and the new poly peptide (polypeptide) is completely moved out into the cytoplasm through a crevice in the large sub-unit. The two ribosome sub-units disengage, split up and are re-used or broken down.

Summary:
- Nearly all the proteins required by cells are synthesised by ribosomes. Ribosomes are found 'free' in the prison cell cytoplasm and besides attached to rough endoplasmic reticulum.
- Ribosomes receive information from the cell nucleus and construction materials from the cytoplasm.
- Ribosomes translate data encoded in messenger ribonucleic acid (mRNA).
- They link together specific amino acids to course polypeptides and they export these to the cytoplasm.
- A mammalian cell may contain as many every bit 10 million ribosomes, but each ribosome has only a temporary existence.
- Ribosomes tin link up amino acids at a rate of 200 per minute.
- Ribosomes are formed from the locking of a pocket-sized sub-unit on to a large sub-unit of measurement. The sub-units are unremarkably available in the cytoplasm, the larger one being about twice the size of the smaller one.
- Each ribosome is a circuitous of ribonucleoproteins with two-thirds of its mass is equanimous of ribosomal RNA and nigh 1-third ribosomal protein.
- Protein production takes place in iii stages: (1) initiation, (2) elongation, and (iii) termination.
- During peptide production the ribosome moves along the mRNA in an intermittent process called translocation.
- Antibiotic drugs such equally streptomycin tin exist used to attack the translation mechanism in prokaryotes. This is very useful. Unfortunately some bacterial toxins and viruses tin too do this.
- Later they get out the ribosome most proteins are folded or modified in some mode. This is chosen 'post translational modification'.
-
An overview diagram of protein production, including a note about protein modification.
Source: https://bscb.org/learning-resources/softcell-e-learning/ribosome/
Post a Comment for "what structures are the bound ribosomes attached to"